Double annulation of ortho- and peri-C–H bonds of fused (hetero)arenes to unusual oxepino-pyridines†
Abstract
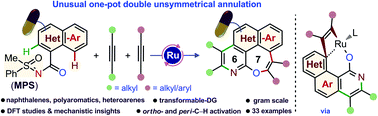
Direct difunctionalization of chemically distinct ortho- and peri-C–H bonds of fused hetero(arenes) is illustrated through an unusual one-pot domino {[4 + 2] & [5 + 2]} double annulation with alkynes for the first time. This process is viable under Ru(II)-catalysis using a sulfoximine directing group and builds four bonds [(C–C)–(C–N) and (C–C)–(C–O)] in a single operation. Such synthetic manifestation offers access to uncommon [6,7]-fused oxepino-pyridine skeletons. DFT calculations provide mechanistic insight into this double annulation of naphthoic acid derivatives with alkynes and corroborate the participation of a ruthena-oxabicyclooctene intermediate, which is responsible for the rare 7-membered ring formation.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...