Synthesis of secondary and tertiary amides without coupling agents from amines and potassium acyltrifluoroborates (KATs)†
Abstract
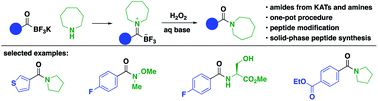
Although highly effective for most amide syntheses, the activation of carboxylic acids requires the use of problematic coupling reagents and is often poorly suited for challenging cases such as N-methyl amino acids. As an alternative to both secondary and tertiary amides, we report their convenient synthesis by the rapid oxidation of trifluoroborate iminiums (TIMs). TIMs are easily prepared by acid-promoted condensation of potassium acyltrifluoroborates (KATs) and amines and are cleanly and rapidly oxidized to amides with hydrogen peroxide. The overall transformation can be conducted either as a one-pot procedure or via isolation of the TIM. The unique nature of the neutral, zwitterionic TIMs makes possible the preparation of tertiary amides via an iminium species that would not be accessible from other carbonyl derivatives and can be conducted in the presence of unprotected functional groups including acids, alcohols and thioethers. In preliminary studies, this approach was applied to the late-stage modifications of long peptides and the iterative synthesis of short, N-methylated peptides without the need for coupling agents.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...