Demonstration of the utility of DOS-derived fragment libraries for rapid hit derivatisation in a multidirectional fashion†
Abstract
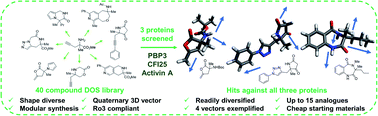
Organic synthesis underpins the evolution of weak fragment hits into potent lead compounds. Deficiencies within current screening collections often result in the requirement of significant synthetic investment to enable multidirectional fragment growth, limiting the efficiency of the hit evolution process. Diversity-oriented synthesis (DOS)-derived fragment libraries are constructed in an efficient and modular fashion and thus are well-suited to address this challenge. To demonstrate the effective nature of such libraries within fragment-based drug discovery, we herein describe the screening of a 40-member DOS library against three functionally distinct biological targets using X-Ray crystallography. Firstly, we demonstrate the importance for diversity in aiding hit identification with four fragment binders resulting from these efforts. Moreover, we also exemplify the ability to readily access a library of analogues from cheap commercially available materials, which ultimately enabled the exploration of a minimum of four synthetic vectors from each molecule. In total, 10–14 analogues of each hit were rapidly accessed in three to six synthetic steps. Thus, we showcase how DOS-derived fragment libraries enable efficient hit derivatisation and can be utilised to remove the synthetic limitations encountered in early stage fragment-based drug discovery.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...