Synthesis of thioethers, arenes and arylated benzoxazoles by transformation of the C(aryl)–C bond of aryl alcohols†
Abstract
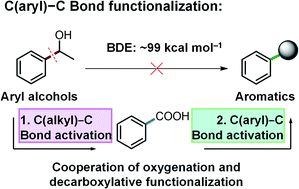
Transformation of aryl alcohols into high-value functionalized aromatic compounds by selective cleavage and functionalization of the C(aryl)–C(OH) bond is of crucial importance, but very challenging by far. Herein, for the first time, we report a novel and versatile strategy for activation and functionalization of C(aryl)–C(OH) bonds by the cooperation of oxygenation and decarboxylative functionalization. A diverse range of aryl alcohol substrates were employed as arylation reagents via the cleavage of C(aryl)–C(OH) bonds and effectively converted into corresponding thioether, arene, and arylated benzoxazole products in excellent yields, in a Cu based catalytic system using O2 as the oxidant. This study offers a new way for aryl alcohol conversion and potentially offers a new opportunity to produce high-value functionalized aromatics from renewable feedstocks such as lignin which features abundant C(aryl)–C(OH) bonds in its linkages.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...