A reconstructed porous copper surface promotes selectivity and efficiency toward C2 products by electrocatalytic CO2 reduction†
Abstract
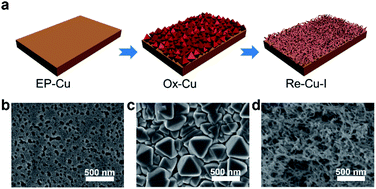
Electrocatalytic synthesis of multicarbon (C2+) products from CO2 reduction suffers from poor selectivity and low energy efficiency. Herein, a facile oxidation–reduction cycling method is adopted to reconstruct the Cu electrode surface with the help of halide anions. The surface composed of entangled Cu nanowires with hierarchical pores is synthesized in the presence of I−, exhibiting a C2 faradaic efficiency (FE) of 80% at −1.09 V vs. RHE. A partial current density of 21 mA cm−2 is achieved with a C2 half-cell power conversion efficiency (PCE) of 39% on this electrode. Such high selective C2 production is found to mainly originate from CO intermediate enrichment inside hierarchical pores rather than the surface lattice effect of the Cu electrode.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...