Expedient synthesis of conjugated triynes via alkyne metathesis†
Abstract
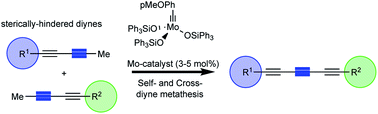
The first synthesis of conjugated triynes by molybdenum-catalysed alkyne metathesis is reported. Strategic to the success of this approach is the utilization of sterically-hindered diynes that allowed for the site-selective alkyne metathesis to produce the desired conjugated triyne products. The steric hindrance of the alkyne moiety was found to be crucial in preventing the formation of diyne byproducts. This novel synthetic strategy was amenable to self- and cross-metathesis providing straightforward access to the corresponding symmetrical and dissymmetrical triynes with high selectivity.
- This article is part of the themed collection: 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...