Speciation of Be2+ in acidic liquid ammonia and formation of tetra- and octanuclear beryllium amido clusters†
Abstract
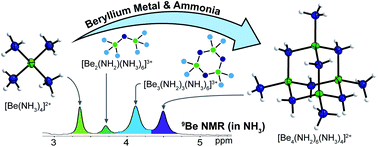
The hexa-μ2-amido-tetraammine-tetraberyllium compounds [Be4(NH2)6(NH3)4]X2 (X = Cl, Br, I, CN, SCN, N3) have been prepared from beryllium metal and NH4X or [Be(NH3)4]X2 in liquid ammonia at ambient temperature. The obtained compounds feature an adamantyl shaped complex cation, which was examined via X-ray diffraction, IR, Raman and NMR spectroscopy. The speciation in solution was studied via15N labeling experiments supplemented with quantum chemistry. Hereby, the intermediates [Be2(NH2)(NH3)6]3+ and [Be3(NH2)3(NH3)6]3+ were identified. Reactivity studies provided bis(N-acetimidoylacetamidinato-N,N′)-beryllium(II), when [Be4(NH2)6(NH3)4]2+ was treated with acetonitrile. While the unprecedented octa-nuclear complex cation [Be8O(NH2)12(C5H5N)4]2+ was received from pyridine. This cluster proves that the [Be4O]6+ core can be stabilized without bidentate O-donor ligands.
- This article is part of the themed collection: 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...