Abstract
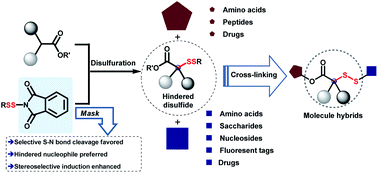
Disulfide bonds are a significant motif in life and drug-delivery systems. In particular, steric hindrance and stereoscopic disulfide linkers are closely associated with the stability of antibody–drug conjugates, which affects the potency, selectivity, and pharmacokinetics of drugs. However, limited availability and diversity of tertiary thiols impede the construction of steric and stereoscopic disulfides for cross-linkage in biochemistry and pharmaceuticals. Through modulating the mask effect of disulfurating reagents, we develop a facile and robust strategy for construction of diverse steric and stereoscopic disulfides via N-dithiophthalimides. The practical cross-linkage of biomolecules including amino acids, saccharides, and nucleosides with different drugs and fluorescent molecules is successfully established through hindered disulfide linkers.


 Please wait while we load your content...
Please wait while we load your content...