Annulative π-extension of BODIPYs made easy via gold(i)-catalyzed cycloisomerization†
Abstract
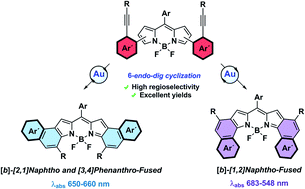
Here we report gold(I)-catalyzed cycloisomerization as a new powerful synthetic tool for the preparation of π-extended BODIPY derivatives. The catalytic system PPhF3AuCl/AgSbF6 enables the synthesis of [b]-[2,1]naphtho-fused-BODIPYs (2a–2c) under mild conditions, in excellent yields and short reaction times. The reaction is totally regioselective to the 6-endo-dig product and for the α-position of the BODIPY, which is both the kinetically and thermodynamically favored pathway, as supported by the free energy profile calculated by means of Density Functional Theory (DFT). Moreover, this methodology also allows the synthesis of two new families of [b]-aryl-fused-BODIPYs, namely, [3,4]phenanthro- (2e and 2f) and [1,2]naphtho-fused (2g) BODIPYs. Their molecular and electronic structures were established by NMR and UV-vis spectroscopies as well as single-crystal X-ray diffraction analysis. As can be noted from the X-ray structures, 2a, 2e and 2g present interesting structural differences at both the molecular and packing level. Interestingly, despite being isomers, the UV/vis spectra of 2a and 2g revealed significant differences in their electronic structures. The origin of this finding was studied by Time-Dependent DFT calculations. Calculated DFT Nuclear Independent Chemical Shift (NICS(0)) values also supported the different electronic structures of 2a and 2g.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...