A bioinspired molybdenum–copper molecular catalyst for CO2 electroreduction†
Abstract
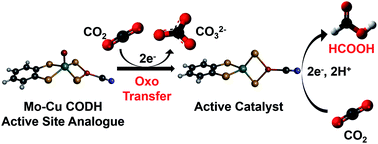
Non-noble metal molecular catalysts mediating the electrocatalytic reduction of carbon dioxide are still scarce. This work reports the electrochemical reduction of CO2 to formate catalyzed by the bimetallic complex [(bdt)MoVI(O)S2CuICN]2− (bdt = benzenedithiolate), a mimic of the active site of the Mo–Cu carbon monoxide dehydrogenase enzyme (CODH2). Infrared spectroelectrochemical (IR-SEC) studies coupled with density functional theory (DFT) computations revealed that the complex is only a pre-catalyst, the active catalyst being generated upon reduction in the presence of CO2. We found that the two-electron reduction of [(bdt)MoVI(O)S2CuICN]2− triggers the transfer of the oxo moiety to CO2 forming CO32− and the complex [(bdt)MoIVS2CuICN]2− and that a further one-electron reduction is needed to generate the active catalyst. Its protonation yields a reactive MoVH hydride intermediate which reacts with CO2 to produce formate. These findings are particularly relevant to the design of catalysts from metal oxo precursors.
- This article is part of the themed collection: Celebrating five years of ChemRxiv


 Please wait while we load your content...
Please wait while we load your content...