Synthesis of azetidines by aza Paternò–Büchi reactions
Abstract
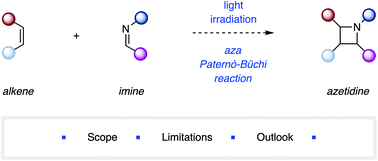
The [2 + 2] photocycloaddition reaction between an imine and an alkene component, the aza Paternò–Büchi reaction, is one of the most efficient ways to synthesize functionalized azetidines. However, the application of the aza Paternò–Büchi reaction has been met with limited success due to the inherent challenges associated with this approach. This review covers the current scope and limitations of reported examples of aza Paternò–Büchi reactions in organic synthesis. An outlook is provided, which highlights recent improvements and the discovery of new reaction protocols that have overcome some long-standing challenges within this field of research.
- This article is part of the themed collections: Most popular 2019-2020 review articles and Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...