A traceless linker for aliphatic amines that rapidly and quantitatively fragments after reduction†
Abstract
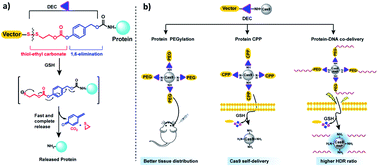
Reduction sensitive linkers (RSLs) have the potential to transform the field of drug delivery due to their ease of use and selective cleavage in intracellular environments. However, despite their compelling attributes, developing reduction sensitive self-immolative linkers for aliphatic amines has been challenging due to their poor leaving group ability and high pKa values. Here a traceless self-immolative linker composed of a dithiol-ethyl carbonate connected to a benzyl carbamate (DEC) is presented, which can modify aliphatic amines and release them rapidly and quantitatively after disulfide reduction. DEC was able to reversibly modify the lysine residues on CRISPR–Cas9 with either PEG, the cell penetrating peptide Arg10, or donor DNA, and generated Cas9 conjugates with significantly improved biological properties. In particular, Cas9–DEC–PEG was able to diffuse through brain tissue significantly better than unmodified Cas9, making it a more suitable candidate for genome editing in animals. Furthermore, conjugation of Arg10 to Cas9 with DEC was able to generate a self-delivering Cas9 RNP that could edit cells without transfection reagents. Finally, conjugation of donor DNA to Cas9 with DEC increased the homology directed DNA repair (HDR) rate of the Cas9 RNP by 50% in HEK 293T cell line. We anticipate that DEC will have numerous applications in biotechnology, given the ubiquitous presence of aliphatic amines on small molecule and protein therapeutics.


 Please wait while we load your content...
Please wait while we load your content...