Sterically controlled reductive oligomerisations of CO by activated magnesium(i) compounds: deltate vs. ethenediolate formation†
Abstract
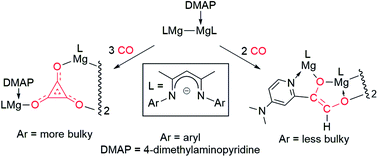
An extremely bulky, symmetrical three-coordinate magnesium(I) complex, [{(TCHPNacnac)Mg}2] (TCHPNacnac = [{(TCHP)NCMe}2CH]−, TCHP = 2,4,6-tricyclohexylphenyl) has been prepared and shown to have an extremely long Mg–Mg bond (3.021(1) Å) for such a complex. It was shown not to react with either DMAP (4-dimethylaminopyridine) or CO. Three unsymmetrical 1 : 1 DMAP adducts of less bulky Mg–Mg bonded species have been prepared, viz. [(ArNacnac)Mg–Mg(DMAP)(ArNacnac)] (ArNacnac = [(ArNCMe)2CH]− Ar = 2,6-xylyl (Xyl), mesityl (Mes) or 2,6-diethylphenyl (Dep)), and their reactivity toward CO explored. Like the previously reported bulkier complex, [(DipNacnac)Mg–Mg(DMAP)(DipNacnac)] (Dip = 2,6-diisopropylphenyl), [(DepNacnac)Mg–Mg(DMAP)(DepNacnac)] reductively trimerises CO to give a rare example of a deltate complex, [{(DepNacnac)Mg(μ-C3O3)Mg(DMAP)(DepNacnac)}2]. In contrast, the two smaller adduct complexes react with only two CO molecules, ultimately giving unusual ethenediolate complexes [{(ArNacnac)Mg{μ-OC(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(DMAP−H)O}Mg(ArNacnac)}2] (Ar = Xyl or Mes). DFT calculations show the latter reactions to proceed via reductive dimerizations of CO, and subsequent intramolecular C–H activation of Mg-ligated DMAP by “zig–zag” [C2O2]2− fragments of reaction intermediates. Calculations also suggest that magnesium deltate complexes are kinetic products in these reactions, while the magnesium ethenediolates are thermodynamic products. This study shows that subtle changes to the bulk of the reacting 1 : 1 DMAP–magnesium(I) adduct complexes can lead to fine steric control over the products arising from their CO reductive oligomerisations. Furthermore, it is found that the more activated nature of the adduct complexes, relative to their symmetrical, three-coordinate counterparts, [{(ArNacnac)Mg}2], likely derives more from the polarisation of the Mg–Mg bonds of the former, than the elongated nature of those bonds.
C(DMAP−H)O}Mg(ArNacnac)}2] (Ar = Xyl or Mes). DFT calculations show the latter reactions to proceed via reductive dimerizations of CO, and subsequent intramolecular C–H activation of Mg-ligated DMAP by “zig–zag” [C2O2]2− fragments of reaction intermediates. Calculations also suggest that magnesium deltate complexes are kinetic products in these reactions, while the magnesium ethenediolates are thermodynamic products. This study shows that subtle changes to the bulk of the reacting 1 : 1 DMAP–magnesium(I) adduct complexes can lead to fine steric control over the products arising from their CO reductive oligomerisations. Furthermore, it is found that the more activated nature of the adduct complexes, relative to their symmetrical, three-coordinate counterparts, [{(ArNacnac)Mg}2], likely derives more from the polarisation of the Mg–Mg bonds of the former, than the elongated nature of those bonds.
- This article is part of the themed collection: Spotlighting main group elements in polynuclear complexes


 Please wait while we load your content...
Please wait while we load your content...