Spin-chemical effects on intramolecular photoinduced charge transfer reactions in bisphenanthroline copper(i)-viologen dyad assemblies†
Abstract
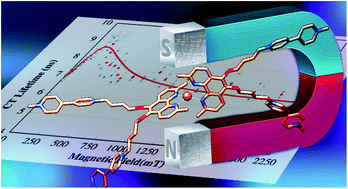
Two covalently linked donor–acceptor copper phenanthroline complexes (C–A dyads) of interest for solar energy conversion/storage schemes, [Cu(I)(Rphen(OMV)24+)2]9+ = RC+A48+ with RC+ = [Cu(I)Rphen2]+ involving 2,9-methyl (R = Me) or 2,9-phenyl (R = Ph)-phenanthroline ligands that are 5,6-disubstituted by 4-(n-butoxy) linked methylviologen electron acceptor groups (A2+ = OMV2+), have been synthesized and investigated via quantum chemical calculations and nanosecond laser flash spectroscopy in 1,2-difluorobenzene/methanol (dfb/MeOH) mixtures. Upon photoexcitation, charge transfer (CT) states RC2+A+A36+ are formed in less than one ns and decay by charge recombination on a time scale of 6–45 ns. The CT lifetime of RC2+A+A36+ has a strong dependence on MeOH solvent fraction when R = Me, but is unaffected if R = Ph. This solvent effect is due to coordination of MeOH solvent in MeC+A48+ (i.e. exciplex formation) allowed by conformational flattening of the ligand sphere, which cannot occur in PhC+A48+ having bulkier Phphen ligand framework. Interestingly, the decay time of the CT state increases for both species at low magnetic fields with a maximum increase of ca. 30% at ca. 150 mT, then decreases as the field is increased up to 1500 mT, the highest field investigated. This magnetic field effect (MFE) is due to magnetic modulation of the spin dynamics interconverting 3CT and 1CT states. A quantitative modeling according to the radical pair mechanism involving ab initio multireference calculations of the complexes revealed that the spin process is dominated by the effect of Cu hyperfine coupling. The external magnetic field suppresses the hyperfine coupling induced spin state mixing thereby lengthening the CT decay time. This effect is counteracted by the field dependent processes of T0–S mixing through the Δg-mechanism and by a local mode spin–orbit mechanism. Further, the maximum MFE is limited by a finite rate of direct recombination of 3CT states and the spin-rotational mechanism of spin relaxation. This study provides a first comprehensive characterization of Cu(II)-complex spin chemistry and highlights how spin chemistry can be used to manipulate solar energy harvesting and storage materials.


 Please wait while we load your content...
Please wait while we load your content...