A polycyclic aromatic hydrocarbon diradical with pH-responsive magnetic properties†
Abstract
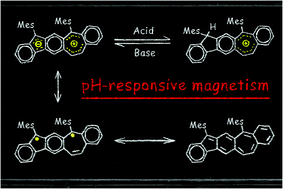
By integrating azulene with a quinoid moiety, a novel non-alternant polycyclic aromatic hydrocarbon molecule BCHF1 exhibiting manifold zwitterionic, quinoidal and diradical behaviors is designed and synthesized. Its zwitterionic feature is evidenced by the changes shown by the 1H-NMR and absorption spectra when the molecule undergoes reversible protonation and deprotonation reactions at varied pH. The diradical facet, manifesting a small singlet–triplet energy gap (ΔES–T), is characterized with a paramagnetic resonance signal detected by the EPR spectroscopy at room temperature. As the diradical properties are not observed in the protonated form, BCHF1+H+, a pH-controlled reversible magnetic switching behavior is illustrated by monitoring the on and off cycles of EPR signals upon successively adding bases and acids to a solution or exposing a thin film of BCHF1+H+ to base vapor followed by acid vapor.


 Please wait while we load your content...
Please wait while we load your content...