An earth-abundant bimetallic catalyst coated metallic nanowire grown electrode with platinum-like pH-universal hydrogen evolution activity at high current density†
Abstract
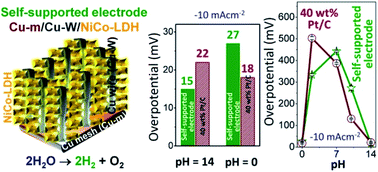
A self-supported and flexible current collector solely made of earth-abundant elements, NiCo layered double hydroxide (LDH) wrapped around Cu nanowires (Cu-Ws) grown on top of commercially available Cu mesh (Cu-m), outperforms the benchmark 40 wt% Pt/C in catalyzing the electrochemical hydrogen evolution reaction (HER). The Cu-m/Cu-W/NiCo-LDH cathode operates both in acidic and alkaline media exhibiting high turnover frequencies (TOF) at 30 mV (0.3 H2 s−1 in 1 M KOH and 0.32 H2 s−1 in 0.5 M H2SO4, respectively) and minimal overpotentials of 15 ± 6 mV in 1 M KOH and 27 ± 2 mV in 0.5 M H2SO4 at −10 mA cm−2. Cu-m/Cu-W/NiCo-LDH outperforms the activity of 40 wt% Pt/C that needs overpotentials of 22 and 18 mV in 1 M KOH and 0.5 M H2SO4, respectively. With a tremendous advantage over Pt/C in triggering proton reduction with fast kinetics, similar mass activity and pH-universality, the current collector demonstrates outstanding operational durability even at above −1 A cm−2. The high density of electronic states near the Fermi energy level of Cu-Ws is found to be a pivotal factor for efficient electron transfer to the NiCo-LDH catalyst. This class of self-supported electrodes is expected to trigger rapid progress in developing high performance energy conversion and storage devices.


 Please wait while we load your content...
Please wait while we load your content...