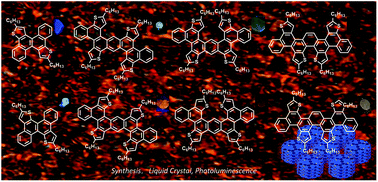
Thiophene-fused polyaromatics: synthesis, columnar liquid crystal, fluorescence and electrochemical properties†
Abstract
Efficient syntheses that incorporate thiophene units into different extended conjugation systems are of interest as a result of the prevalence of sulfur-rich aromatics in organic electronics. Self-organization by using liquid crystal properties is also desirable for optimal processing of organic electronics and optical devices. In this article, we describe a two-step process to access extended regioisomers of polyaromatics with different shapes. This method involves an efficient single or double benzannulation from an alkyne precursor followed by Scholl cyclization. In spite of their unconventional nondiscoid shape, these materials display stable columnar liquid crystal phases. We examine the photophysical and electrochemical properties and find that structurally very similar thiophene-fused polyaromatics display significant differences in their properties.


 Please wait while we load your content...
Please wait while we load your content...