Shape-adaptive single-molecule magnetism and hysteresis up to 14 K in oxide clusterfullerenes Dy2O@C72 and Dy2O@C74 with fused pentagon pairs and flexible Dy–(μ2-O)–Dy angle†
Abstract
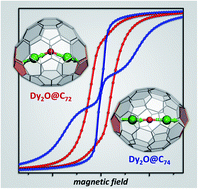
Dysprosium oxide clusterfullerenes Dy2O@Cs(10528)–C72 and Dy2O@C2(13333)–C74 are synthesized and characterized by single-crystal X-ray diffraction. Carbon cages of both molecules feature two adjacent pentagon pairs. These pentalene units determine positions of endohedral Dy ions hence the shape of the Dy2O cluster, which is bent in Dy2O@C72 but linear in Dy2O@C74. Both compounds show slow relaxation of magnetization and magnetic hysteresis. Nearly complete cancelation of ferromagnetic dipolar and antiferromagnetic exchange Dy⋯Dy interactions leads to unusual magnetic properties. Dy2O@C74 exhibits zero-field quantum tunneling of magnetization and magnetic hysteresis up to 14 K, the highest temperature among Dy-clusterfullerenes.


 Please wait while we load your content...
Please wait while we load your content...