Tandem sequential catalytic enantioselective synthesis of highly-functionalised tetrahydroindolizine derivatives†‡
Abstract
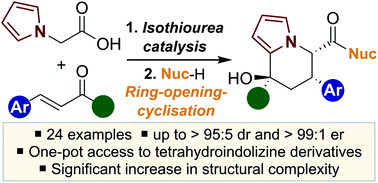
An isothiourea-catalysed enantioselective synthesis of novel tetrahydroindolizine derivatives is reported through a one-pot tandem sequential process. The application of 2-(pyrrol-1-yl)acetic acid in combination with either a trifluoromethyl enone or an α-keto-β,γ-unsaturated ester in an enantioselective Michael addition–lactonisation process, followed by in situ ring-opening and cyclisation, led to a range of 24 tetrahydroindolizine derivatives containing three stereocentres in up to >95 : 5 dr and >99 : 1 er.


 Please wait while we load your content...
Please wait while we load your content...