Non-covalent allosteric regulation of capsule catalysis†
Abstract
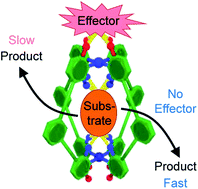
Allosteric regulation is an essential biological process that allows enzymes to modulate their active site properties by binding a control molecule at the protein exterior. Here we show the first example of capsule catalysis in which activity is changed by exotopic binding. This study utilizes a simple Pd2L4 capsule that can partition substrates and external effectors with high fidelity. We also present a detailed, quantitative understanding of how effector interactions alter both substrate and transition state binding. Unlike other allosteric host systems, perturbations are not a consequence of large mechanical changes, rather subtle electronic effects resulting from weak, non-covalent binding to the exterior surface. This investigation paves the way to more sophisticated allosteric systems.


 Please wait while we load your content...
Please wait while we load your content...