Electrostatically-directed Pd-catalysis in combination with C–H activation: site-selective coupling of remote chlorides with fluoroarenes and fluoroheteroarenes†
Abstract
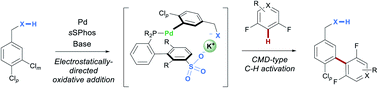
Systems incorporating catalyst–substrate non-covalent interactions are emerging as a versatile approach to address site-selectivity challenges in remote functionalization reactions. Given the achievements that have been made in this regard using metals such as iridium, manganese and rhodium, it is surprising that non-covalent catalyst direction has not been utilized in reactions incorporating palladium-catalyzed C–H activation steps, despite palladium being arguably the most versatile metal for C–H activation. Herein, we demonstrate that electrostatically directed, site-selective C–Cl oxidative addition is compatible with a subsequent C–H activation step, proceeding via a concerted metalation deprotonation-type mechanism. This results in site-selective cross-coupling of dichloroarenes with fluoroarenes and fluoroheteroarenes, with selectivity controlled by catalyst structure. This study demonstrates that Pd-catalyzed C–H activation can be used productively in combination with a non-covalently-directed mode of catalysis, with important implications in both fields.


 Please wait while we load your content...
Please wait while we load your content...