Modular bioengineered kinase sensors via scaffold protein-mediated split-luciferase complementation†
Abstract
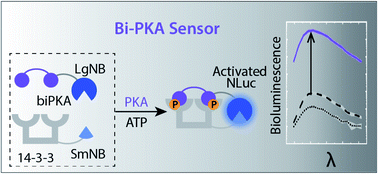
Phosphorylation is a key regulation event in cellular signaling. Sensing the underlying kinase activity is of crucial importance for its fundamental understanding and for drug development. For this, modular kinase activity sensing concepts are urgently needed. We engineered modular serine kinase sensors based on complementation of split NanoBiT luciferase on protein assembly platforms generated from the scaffold protein 14-3-3. The bioengineered platforms are modular and easy adaptable as exemplary shown using novel sensors for the kinases PKA, PKB, and CHK1. Two designs were conceptualized, both relying on binding of defined mono- or bivalent kinase recognition motifs to the 14-3-3 platform upon phosphorylation, resulting in reconstitution of active split-luciferase. Especially the design based on double phosphorylation and bivalent 14-3-3 binding exhibits high efficiency for signal amplification (>1000-fold) and sensitivity to specific kinases, including in cellular lysates.


 Please wait while we load your content...
Please wait while we load your content...