Photoactive preorganized subphthalocyanine-based molecular tweezers for selective complexation of fullerenes†
Abstract
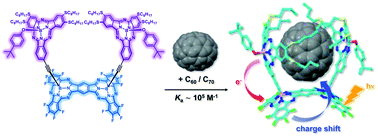
The development of new chromophoric receptors capable of binding curved carbon nanostructures is central to the quest for improved fullerene-based organic photovoltaics. We herein report the synthesis and characterization of a subphthalocyanine-based multicomponent ensemble consisting of two electron-rich SubPc-monomers rigidly attached to the convex surface of an electron-poor SubPc-dimer. Such a unique configuration, especially in terms of the two SubPc-monomers, together with the overall stiffness of the linker, endows the multicomponent system with a well-defined tweezer-like topology to efficiently embrace a fullerene in its inner cavity. The formation of a 1 : 1 complex was demonstrated in a variety of titration studies with either C60 or C70. In solution, the underlying association constants were of the order of 105 M−1. Detailed physicochemical experiments revealed a complex scenario of energy- and electron-transfer processes upon photoexcitation in the absence and presence of fullerenes. The close proximity of the fullerenes to the electron-rich SubPcs enables a charge shift from the initially formed reduced SubPc-dimer to either C60 to C70.
- This article is part of the themed collection: The Mechanics of Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...