Desymmetrization of unactivated bis-alkenes via chiral Brønsted acid-catalysed hydroamination†
Abstract
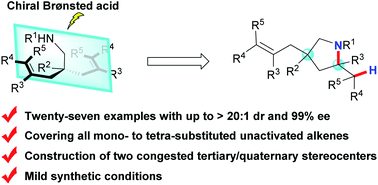
Although great success has been achieved in catalytic asymmetric hydroamination of unactivated alkenes using transition metal catalysis and organocatalysis, the development of catalytic desymmetrising hydroamination of such alkenes remains a tough challenge in terms of attaining a high level of stereocontrol over both remote sites and reaction centers at the same time. To address this problem, here we report a highly efficient and practical desymmetrising hydroamination of unactivated alkenes catalysed by chiral Brønsted acids with both high diastereoselectivity and enantioselectivity. This method features a remarkably broad alkene scope, ranging from mono-substituted and gem-/1,2-disubstituted to the challenging tri- and tetra-substituted alkenes, to provide access to a variety of diversely functionalized chiral pyrrolidines bearing two congested tertiary or quaternary stereocenters with excellent efficiency under mild and user-friendly synthetic conditions. The key to success is indirect activation of unactivated alkenes by chiral Brønsted acids via a concerted hydroamination mechanism.


 Please wait while we load your content...
Please wait while we load your content...