Novel NIR-II organic fluorophores for bioimaging beyond 1550 nm†
Abstract
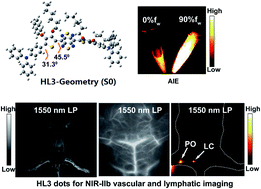
Near-infrared fluorescence imaging in the 1500–1700 nm sub-window (NIR-IIb) has shown a deeper penetration depth, higher resolution and zero auto-fluorescence for biomedical imaging. Till now, very few small molecule NIR-IIb fluorophores have been reported due to the extremely rare organic NIR-IIb skeleton and a notorious aggregation-caused quenching (ACQ) effect in aqueous solution. In this study, highly twisted NIR-II small molecule fluorophores such as HL3 (45.5° at the S0 state) with the emission wavelength extending into the NIR-IIb region were designed and synthesized using an aggregation-induced emission (AIE) strategy. HL3 dots showed a remarkable increase in fluorescence intensity with a QY of 11.7% in the NIR-II window (>1000 nm) and 0.05% in the NIR-IIb region (>1550 nm) in water. High-resolution in vivo imaging of the whole body, cerebral vasculature, and lymphatic drainage beyond 1550 nm was achieved using NIR-II AIE HL3 dots for the first time. These attractive results may promote the development of small-molecule NIR-IIb fluorophores with the maximum emission wavelength beyond 1500 nm with a deeper penetration depth and higher resolution.
- This article is part of the themed collections: In celebration of Chinese New Year and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...