The redox-coupled proton-channel opening in cytochrome c oxidase†
Abstract
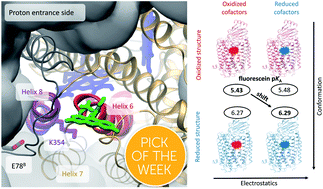
Cytochrome c oxidase (CcO), a redox-coupled proton pump, catalyzes the reduction of molecular oxygen to water, thereby establishing the transmembrane proton gradient that fuels ATP synthesis. CcO employs two channels for proton uptake, the D- and the K-channel. In contrast to the D-channel, the K-channel does not constitute a continuous pathway of H-bonds for proton conduction and is only active in the reductive phase rendering its proton transport mechanism enigmatic. Theoretical studies have suggested selective hydration changes within the K-channel to become activated and being essential for vectorial proton transport. Here, we unravel a previously unidentified mechanism for transient proton channel activation by combining computational studies with site-directed nano-environmental probing of protonation, structural changes, and water dynamics. We show that electrostatic changes at the binuclear center lead to long-range conformational changes propagating to the K-channel entrance as evidenced by time-resolved fluorescence depolarization experiments and molecular dynamics (MD) simulations. These redox-induced long-range structural rearrangements affect the H-bond network at the K-channel's protein surface as shown by pKa-shift analysis of a local probe in experiment and simulation. Concomitantly, selective channel hydration at the K-channel entrance was revealed by dipolar relaxation studies to be associated with channel opening. We propose that instead of a singular change, it is the intricate interplay of these individual redox-triggered changes in the cause–effect relationship that defines the mechanism for transient proton conduction of the K-channel.
- This article is part of the themed collections: 2020 ChemSci Pick of the Week Collection and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...