Direct cyclopropanation of activated N-heteroarenes via site- and stereoselective dearomative reactions†
Abstract
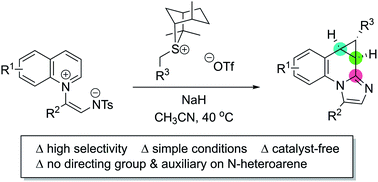
A divergent cyclopropanation reaction has been accomplished via the dearomative addition of sulfur ylides to activated N-heteroarenes. A series of biologically significant molecular skeletons was obtained by the direct cyclopropanation of quinolinium zwitterions. Furthermore, a straightforward synthetic route to optically enriched cyclopropane-fused heterocycles was developed using sulfur ylides as chiral nucleophiles in the 1,4-dearomative reaction.


 Please wait while we load your content...
Please wait while we load your content...