Examining the role of phosphorylation of p19INK4d in its stability and ubiquitination using chemical protein synthesis†
Abstract
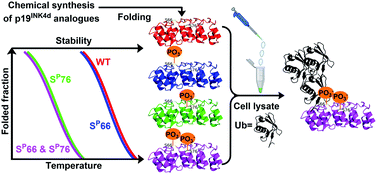
p19INK4d plays an important role in the regulation of the cell cycle by inhibiting the function of cyclin-dependent kinases 4/6 that is responsible for the phosphorylation and deactivation of the retinoblastoma protein (pRb) tumour suppressor. Recently, it was reported that phosphorylation of p19INK4d at Ser76 and Ser66 causes structural changes, which lead to its ubiquitination and degradation. Yet the exact contribution of each phosphorylation site remains unclear. To shed light on the role of these sites, we developed the chemical synthesis of unmodified, mono- and doubly phosphorylated p19INK4d using state of the art methods for chemical protein synthesis. The synthesized proteins were characterized by circular dichroism and biochemical methods to examine the effect of phosphorylation on the thermal stability and ubiquitination, respectively. Our results provide clear determination of p19INK4d stability upon phosphorylation at different sites and reveal that phosphorylation of both Ser residues might be necessary for promoting ubiquitination of p19INK4d.


 Please wait while we load your content...
Please wait while we load your content...