Radical cascade synthesis of azoles via tandem hydrogen atom transfer†
Abstract
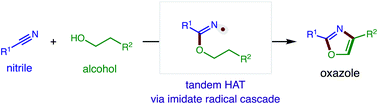
A radical cascade strategy for the modular synthesis of five-membered heteroarenes (e.g. oxazoles, imidazoles) from feedstock reagents (e.g. alcohols, amines, nitriles) has been developed. This double C–H oxidation is enabled by in situ generated imidate and acyloxy radicals, which afford regio- and chemo-selective β C–H bis-functionalization. The broad synthetic utility of this tandem hydrogen atom transfer (HAT) approach to access azoles is included, along with experiments and computations that provide insight into the selectivity and mechanism of both HAT events.


 Please wait while we load your content...
Please wait while we load your content...