The enantioselective total synthesis of laurendecumallene B†
Abstract
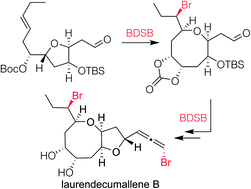
For decades, the Laurencia family of halogenated C15-acetogenins has served as a valuable testing ground for the prowess of chemical synthesis, particularly as it relates to generating functionalized 8-membered bromoethers. Herein, we show that a readily modified and predictable approach that generates such rings and an array of attendant stereocenters via a bromenium-induced cyclization/ring-expansion process can be used to synthesize laurendecumallene B and determine the configuration of two of its previously unassigned stereocenters. In particular, this work highlights how the use of the bromenium source BDSB (Et2SBr·SbCl5Br) in non-conventional solvents is essential in generating much of the target's complexity in optimal yields and stereoselectivity. Moreover, the final structural assignment of laurendecumallene B reveals that it has one element of bromine-based chirality that, to the best of our knowledge, is not shared with any other member of the class.


 Please wait while we load your content...
Please wait while we load your content...