Regio- and diastereoselective reactions of chiral secondary alkylcopper reagents with propargylic phosphates: preparation of chiral allenes†
Abstract
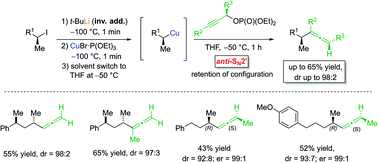
The diastereoselective SN2′-substitution of secondary alkylcopper reagents with propargylic phosphates enables the preparation of stereodefined alkylallenes. By using enantiomerically enriched alkylcopper reagents and enantioenriched propargylic phosphates as electrophiles anti-SN2′-substitutions were performend leading to α-chiral allenes in good yields with excellent regioselectivity and retention of configuration. DFT-calculations were performed to rationalize the structure of these alkylcopper reagents in various solvents, emphasizing their configurational stability in THF.


 Please wait while we load your content...
Please wait while we load your content...