Base induced isomerisation of a phosphaethynolato-borane: mechanistic insights into boryl migration and decarbonylation to afford a triplet phosphinidene†
Abstract
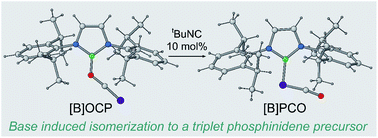
We report on the (tert-butyl)isocyanide-catalysed isomersation of a phosphaethynolato-borane, [B]OCP ([B] = N,N′-bis(2,6-diisopropylphenyl)-2,3-dihydro-1H-1,3,2-diazaboryl), to its linkage isomer, a phosphaketenyl-borane, [B]PCO. Mechanistic insight into this unusual isomerisation was gained through a series of stoichiometric reactions of [B]OCP with isocyanides and theoretical calculations at the Density Functional Theory (DFT) level. [B]PCO decarbonylates under photolytic conditions to afford a novel boryl-substituted diphosphene, [B]P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P[B]. This reaction proceeds via a transient triplet phosphinidene which we have been able to observe spectroscopically by Electron Paramagnetic Resonance (EPR) spectroscopy.
P[B]. This reaction proceeds via a transient triplet phosphinidene which we have been able to observe spectroscopically by Electron Paramagnetic Resonance (EPR) spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...