Transition metal-free catalytic reduction of primary amides using an abnormal NHC based potassium complex: integrating nucleophilicity with Lewis acidic activation†
Abstract
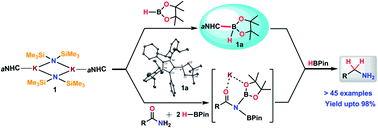
An abnormal N-heterocyclic carbene (aNHC) based potassium complex was used as a transition metal-free catalyst for reduction of primary amides to corresponding primary amines under ambient conditions. Only 2 mol% loading of the catalyst exhibits a broad substrate scope including aromatic, aliphatic and heterocyclic primary amides with excellent functional group tolerance. This method was applicable for reduction of chiral amides and utilized for the synthesis of pharmaceutically valuable precursors on a gram scale. During mechanistic investigation, several intermediates were isolated and characterized through spectroscopic techniques and one of the catalytic intermediates was characterized through single-crystal XRD. A well-defined catalyst and isolable intermediate along with several stoichiometric experiments, in situ NMR experiments and the DFT study helped us to sketch the mechanistic pathway for this reduction process unravelling the dual role of the catalyst involving nucleophilic activation by aNHC along with Lewis acidic activation by K ions.


 Please wait while we load your content...
Please wait while we load your content...