Solar water splitting over Rh0.5Cr1.5O3-loaded AgTaO3 of a valence-band-controlled metal oxide photocatalyst†
Abstract
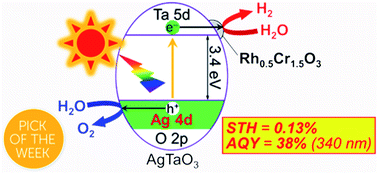
Improvement of water splitting performance of AgTaO3 (BG 3.4 eV) of a valence-band-controlled photocatalyst was examined. Survey of cocatalysts revealed that a Rh0.5Cr1.5O3 cocatalyst was much more effective than Cr2O3, RuO2, NiO and Pt for water splitting into H2 and O2 in a stoichiometric amount. The optimum loading amount of the Rh0.5Cr1.5O3 cocatalyst was 0.2 wt%. The apparent quantum yield (AQY) at 340 nm of the optimized Rh0.5Cr1.5O3(0.2 wt%)/AgTaO3 photocatalyst reached to about 40%. Rh0.5Cr1.5O3(0.2 wt%)/AgTaO3 gave a solar to hydrogen conversion efficiency (STH) of 0.13% for photocatalytic water splitting under simulated sunlight irradiation. Bubbles of gasses evolved by the solar water splitting were visually observed under atmospheric pressure at room temperature.
- This article is part of the themed collections: 2020 ChemSci Pick of the Week Collection and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...