Cross-dehydrogenative coupling enables enantioselective access to CF3-substituted all-carbon quaternary stereocenters†
Abstract
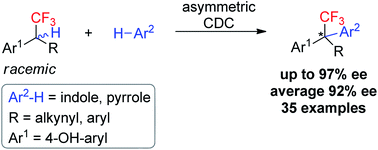
A cross-dehydrogenative coupling strategy for enantioselective access to acyclic CF3-substituted all-carbon quaternary stereocenters has been established. By using catalytic DDQ with MnO2 as an inexpensive terminal oxidant, asymmetric cross coupling of racemic δ-CF3-substituted phenols with indoles proceeded smoothly, providing CF3-bearing all-carbon quaternary stereocenters with excellent chemo- and enantioselectivities. The generality of the strategy is further demonstrated by efficient construction of all-carbon quaternary stereocenters bearing other polyfluoroalkyl and perfluoroalkyl groups such as CF2Cl, C2F5, and C3F7.


 Please wait while we load your content...
Please wait while we load your content...