Isolation of singlet carbene derived 2-phospha-1,3-butadienes and their sequential one-electron oxidation to radical cations and dications†
Abstract
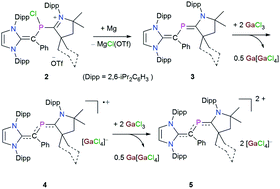
A synthetic strategy for the 2-phospha-1,3-butadiene derivatives [{(IPr)C(Ph)}P(cAACMe)] (3a) and [{(IPr)C(Ph)}P(cAACCy)] (3b) (IPr = C{(NDipp)CH}2, Dipp = 2,6-iPr2C6H3; cAACMe = C{(NDipp)CMe2CH2CMe2}; cAACCy = C{(NDipp)CMe2CH2C(Cy)}, Cy = cyclohexyl) containing a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–P
C–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C framework has been established. Compounds 3a and 3b have a remarkably small HOMO–LUMO energy gap (3a: 5.09; 3b: 5.05 eV) with a very high-lying HOMO (−4.95 eV for each). Consequently, 3a and 3b readily undergo one-electron oxidation with the mild oxidizing agent GaCl3 to afford radical cations [{(IPr)C(Ph)}P(cAACR)]GaCl4 (R = Me 4a, Cy 4b) as crystalline solids. The main UV-vis absorption band for 4a and 4b is red-shifted with respect to that of 3a and 3b, which is associated with the SOMO related transitions. The EPR spectra of compounds 4a and 4b each exhibit a doublet due to coupling of the unpaired electron with the 31P nucleus. Further one-electron removal from the radical cations 4a and 4b is also feasible with GaCl3, affording the dications [{(IPr)C(Ph)}P(cAACR)](GaCl4)2 (R = Me 5a, Cy 5b) as yellow crystals. The molecular structures of compounds 3–5 have been determined by X-ray diffraction and analyzed by DFT calculations.
C framework has been established. Compounds 3a and 3b have a remarkably small HOMO–LUMO energy gap (3a: 5.09; 3b: 5.05 eV) with a very high-lying HOMO (−4.95 eV for each). Consequently, 3a and 3b readily undergo one-electron oxidation with the mild oxidizing agent GaCl3 to afford radical cations [{(IPr)C(Ph)}P(cAACR)]GaCl4 (R = Me 4a, Cy 4b) as crystalline solids. The main UV-vis absorption band for 4a and 4b is red-shifted with respect to that of 3a and 3b, which is associated with the SOMO related transitions. The EPR spectra of compounds 4a and 4b each exhibit a doublet due to coupling of the unpaired electron with the 31P nucleus. Further one-electron removal from the radical cations 4a and 4b is also feasible with GaCl3, affording the dications [{(IPr)C(Ph)}P(cAACR)](GaCl4)2 (R = Me 5a, Cy 5b) as yellow crystals. The molecular structures of compounds 3–5 have been determined by X-ray diffraction and analyzed by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...