Concerted proton-electron transfer oxidation of phenols and hydrocarbons by a high-valent nickel complex†
Abstract
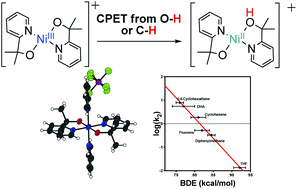
The high-valent nickel(III) complex Ni(pyalk)2+ (2) was prepared by oxidation of a nickel(II) complex, Ni(pyalk)2 (1) (pyalk = 2-pyridyl-2-propanoate). 2 and derivatives were fully characterized by mass spectrometry and X-ray crystallography. Electron paramagnetic resonance spectroscopy and X-ray photoelectron spectroscopy confirm that the oxidation is metal-centered. 2 was found to react with a variety of phenolic and hydrocarbon substrates. A linear correlation between the measured rate constant and the substrate bond dissociation enthalpy (BDE) was found for both phenolic and hydrocarbon substrates. Large H/D kinetic isotope effects were also observed for both sets of substrates. These results suggest that 2 reacts through concerted proton-electron transfer (CPET). Analysis of measured thermodynamic parameters allows us to calculate a bond dissociation free energy (BDFE) of ∼91 kcal mol−1 for the O–H bond of the bound pyalk ligand. These findings may shed light onto CPET steps in oxidative catalysis and have implications for ligand design in catalytic systems.
- This article is part of the themed collection: Papers celebrating the birthday and achievements of Professor Michael Wasielewski


 Please wait while we load your content...
Please wait while we load your content...