A new hypervalent iodine(iii/v) oxidant and its application to the synthesis of 2H-azirines†
Abstract
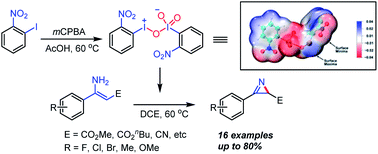
The reaction of o-nitroiodobenzene and mCPBA in acetic acid was found to afford a novel hypervalent iodine compound, in the structure of which both iodine(III) and iodine(V) moieties coexist. The nitro groups at the ortho phenyl positions were found to be crucial in stabilizing this uncommon structure. This novel hypervalent iodine(III/V) oxidant is proved to be effective in realizing the synthesis of 2-unsubstitued 2H-azirines via intramolecular oxidative azirination, which could not be efficiently achieved by the existing known hypervalent iodine reagents.


 Please wait while we load your content...
Please wait while we load your content...