Automated linkage of proteins and payloads producing monodisperse conjugates†
Abstract
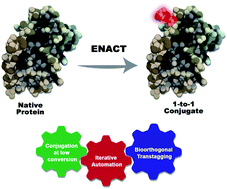
Controlled protein functionalization holds great promise for a wide variety of applications. However, despite intensive research, the stoichiometry of the functionalization reaction remains difficult to control due to the inherent stochasticity of the conjugation process. Classical approaches that exploit peculiar structural features of specific protein substrates, or introduce reactive handles via mutagenesis, are by essence limited in scope or require substantial protein reengineering. We herein present equimolar native chemical tagging (ENACT), which precisely controls the stoichiometry of inherently random conjugation reactions by combining iterative low-conversion chemical modification, process automation, and bioorthogonal trans-tagging. We discuss the broad applicability of this conjugation process to a variety of protein substrates and payloads.


 Please wait while we load your content...
Please wait while we load your content...