Aldehydes and ketones influence reactivity and selectivity in nickel-catalysed Suzuki–Miyaura reactions†‡§
Abstract
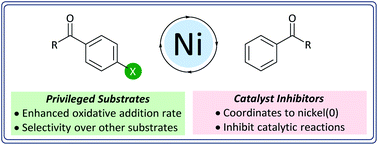
The energetically-favorable coordination of aldehydes and ketones – but not esters or amides – to Ni0 during Suzuki–Miyaura reactions can lead either to exquisite selectivity and enhanced reactivity, or to inhibition of the reaction. Aryl halides where the C–X bond is connected to the same π-system as an aldehyde or ketone undergo unexpectedly rapid oxidative addition to [Ni(COD)(dppf)] (1), and are selectively cross-coupled during competition reactions. When aldehydes and ketones are present in the form of exogenous additives, the cross-coupling reaction is inhibited to an extent that depends on the strength of the coordination of the pendant carbonyl group to Ni0. This work advances our understanding of how common functional groups interact with Ni0 catalysts and how these interactions affect workhorse catalytic reactions in academia and industry.


 Please wait while we load your content...
Please wait while we load your content...