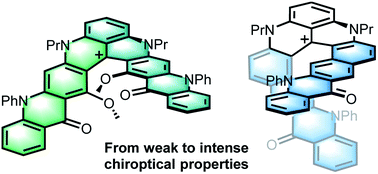
Merging polyacenes and cationic helicenes: from weak to intense chiroptical properties in the far red region†
Abstract
A series of helical tetracenes and pentacenes was synthesized from cationic [6] and [4]helicene precursors. These colorful acenes fluoresce in the far red region. While [4]helicene-based pentacenes exhibit chiroptical properties mainly in the UV region, [6]helicene-derived tetracenes show enhanced ECD in the visible range, in addition to clear CPL responses. This difference is rationalized using first principles.


 Please wait while we load your content...
Please wait while we load your content...