Abstract
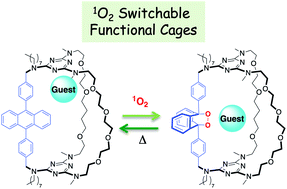
Molecular cages 1a and 2a incorporating a 9,10-diphenylanthracene (DPA) chromophore were synthesized through a templated ring-closure metathesis approach that allows variation in cavity size through the introduction of up to three different pillars. Reversible Diels–Alder reaction between the DPA moiety and photogenerated singlet oxygen smoothly converted 1a and 2a to the corresponding endoperoxide cages 1b and 2b, which are converted back to 1a and 2a upon heating. Endoperoxide formation constitutes a reversible covalent signal that combines structural changes in the interior of the cage with introduction of two additional coordination sites. This results in a large modulation of the binding ability of the receptors attributed to a change in the location of the preferred binding site owing to the added coordination by the endoperoxide oxygen lone pairs. Cages 1a and 2a form complexes with sodium and cesium whose association constants are modified by 4–20 fold for Na+ and 200–450 fold for Cs+ upon conversion to 1b and 2b. DFT calculations show that in the anthracene form, cages 1a and 2a can bind 2 metal cations in their periphery so that each cation is coordinated by 4 oxygens and one amine nitrogen, whereas the endoperoxide cages 1b and 2b bind cations centrally in a geometry that favors coordination to the endoperoxide oxygens.


 Please wait while we load your content...
Please wait while we load your content...