Trisulfur radical anion-triggered stitching thienannulation: rapid access to largely π-extended thienoacenes†
Abstract
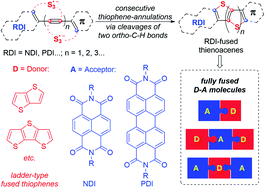
Largely π-extended rylene diimide-fused thienoacenes, a new family of fully fused electron donor–acceptor (D–A) molecules, have been readily synthesized by a novel trisulfur radical anion (S3˙−)-triggered stitching thienannulation strategy. The ladder-type fused thiophene cores are constructed in a stitching manner through multiple carbon–sulfur bond formation between acetylenic rylene dyes and S3˙−. A detailed mechanistic study of these stitching thienannulations unveiled the multiple reactivities of S3˙−. Physical properties of the newly formed D–A, A–D–A, and D–A–D type thienoacenes have also been investigated, which revealed their precisely controllable electronic properties.
- This article is part of the themed collection: Celebrating a Century of Excellency in Chemistry at Xiamen University


 Please wait while we load your content...
Please wait while we load your content...