Catalytic asymmetric multiple dearomatizations of phenols enabled by a cascade 1,8-addition and Diels–Alder reaction†
Abstract
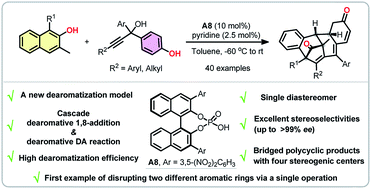
A direct catalytic asymmetric multiple dearomatization reaction of phenols was disclosed, which provides expedient access to a series of architecturally complex polycyclic compounds bearing four stereogenic centers in high enantiopurity. The key to achieve such a transformation is the combination of a dearomative 1,8-addition of β-naphthols to para-quinone methides generated in situ from propargylic alcohols and a subsequent intramolecular dearomative Diels–Alder reaction. Noteworthily, this protocol enrichs not only the diversity of dearomatized products but also the toolbox of dearomatization strategies.


 Please wait while we load your content...
Please wait while we load your content...