Probing enzymatic activity – a radical approach†
Abstract
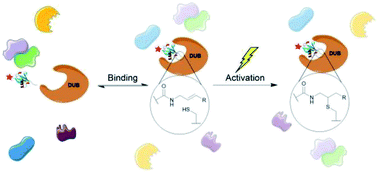
Deubiquitinating enzymes (DUBs) are known to have numerous important interactions with the ubiquitin cascade and their dysregulation is associated with several diseases, including cancer and neurodegeneration. They are an important class of enzyme, and activity-based probes have been developed as an effective strategy to study them. Existing activity-based probes that target the active site of these enzymes work via nucleophilic mechanisms. We present the development of latent ubiquitin-based probes that target DUBs via a site selective, photoinitiated radical mechanism. This approach differs from existing photocrosslinking probes as it requires a free active site cysteine. In contrast to existing cysteine reactive probes, control over the timing of the enzyme–probe reaction is possible as the alkene warhead is completely inert under ambient conditions, even upon probe binding. The probe's reactivity has been demonstrated against recombinant DUBs and to capture endogenous DUB activity in cell lysate. This allows more finely resolved investigations of DUBs.
- This article is part of the themed collection: Most popular 2019-2020 chemical biology articles


 Please wait while we load your content...
Please wait while we load your content...