Breaking scaling relations for efficient CO2 electrochemical reduction through dual-atom catalysts†
Abstract
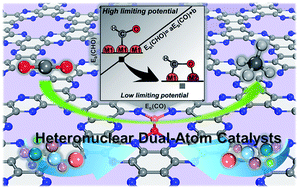
The electrochemical reduction of CO2 offers an elegant solution to the current energy crisis and carbon emission issues, but the catalytic efficiency for CO2 reduction is seriously restricted by the inherent scaling relations between the adsorption energies of intermediates. Herein, by combining the concept of single-atom catalysts and multiple active sites, we design heteronuclear dual-atom catalysts to break through the stubborn restriction of scaling relations on catalytic activity. Twenty-one kinds of heteronuclear transition-metal dimers are embedded in monolayer C2N as potential dual-atom catalysts. First-principles calculations reveal that by adjusting the components of dimers, the two metal atoms play the role of carbon adsorption sites and oxygen adsorption sites respectively, which results in the decoupling of adsorption energies of key intermediates. Free energy profiles demonstrate that CO2 can be efficiently reduced to CH4 on CuCr/C2N and CuMn/C2N with low limiting potentials of −0.37 V and −0.32 V, respectively. This study suggests that the introduction of multiple active sites into porous two-dimensional materials would provide a great possibility for breaking scaling relations to achieve efficient multi-intermediate electrocatalytic reactions.
- This article is part of the themed collections: In celebration of Chinese New Year and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...