Development of copper-catalyzed enantioselective decarboxylative aldolization for the preparation of perfluorinated 1,3,5-triols featuring supramolecular recognition properties†
Abstract
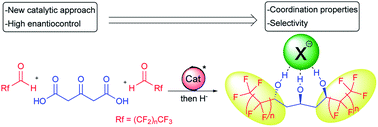
Fluorine is able to confer unique properties to organic molecules but the scarcity of natural organofluorine sources renders the development of new synthetic methods highly desirable. Using a chiral BOX/Cu combination, enantioselective decarboxylative aldolization of perfluorinated aldehydes has been developed. Most notably, the reaction occurring under mild conditions and with high enantiocontrol can create ketodiols in one single synthetic operation, which are precursors of crucial perfluorinated 1,3,5-triols. In addition, the reaction performed with chloral, validates the proposed transition state model based on steric interactions and provides the first enantioselective synthesis of hexachlorinated ketodiol of great synthetic utility. The ability of perfluorinated 1,3,5-triols to form a central hydrogen-bonding framework allows strong coordination of anions and the chirality obtained through the catalyst-controlled synthetic sequence demonstrates the selective chiral anion recognition ability of polyols.


 Please wait while we load your content...
Please wait while we load your content...