Hydrogen and proton exchange at carbon. Imbalanced transition state and mechanism crossover†
Abstract
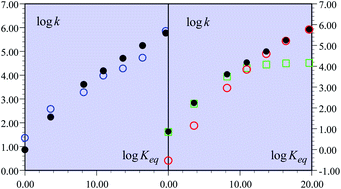
A recent remarkable study of the C–H oxidation of substituted fluorenyl-benzoates together with the transfer of a proton to an internal receiving group by means of electron transfer outer-sphere oxidants, in the noteworthy absence of hydrogen-bonding interactions, is taken as an example to uncover the existence of a mechanism crossover, making the reaction pass from a CPET pathway to a PTET pathway as the driving force of the global reaction decreases. This was also the occasion to stress that considerations based on “imbalanced” or “asynchronous” transition states cannot replace activation/driving force models based on the quantum mechanical treatment of both electrons and transferring protons.


 Please wait while we load your content...
Please wait while we load your content...