Unravelling the mechanism of cobalt-catalysed remote C–H nitration of 8-aminoquinolinamides and expansion of substrate scope towards 1-naphthylpicolinamide†
Abstract
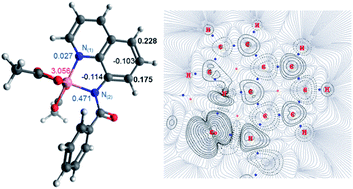
Previously, an unexpected Co-catalysed remote C–H nitration of 8-aminoquinolinamide compounds was developed. This report provided a novel reactivity for Co which was assumed to proceed through the mechanistic pathway already known for analogous Cu-catalysed remote couplings of the same substrates. In order to shed light into this intriguing, and previously unobserved reactivity for Co, a thorough computational study has now been performed, which has allowed for a full understanding of the operative mechanism. This study demonstrates that the Co-catalysed remote coupling does not occur through the previously proposed Single Electron Transfer (SET) mechanism, but actually operates through a high-spin induced remote radical coupling mechanism, through a key intermediate with significant proportion of spin density at the 5- and 7-positions of the aminoquinoline ring. Additionally, new experimental data provides expansion of the synthetic utility of the original nitration procedure towards 1-naphthylpicolinamide which unexpectedly appears to operate via a subtly different mechanism despite having a similar chelate environment.


 Please wait while we load your content...
Please wait while we load your content...