Reduction of a dihydroboryl cation to a boryl anion and its air-stable, neutral hydroboryl radical through hydrogen shuttling†
Abstract
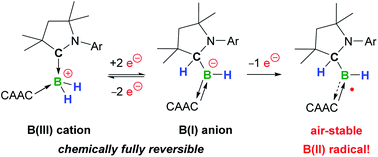
The addition of Lewis bases to a cyclic (alkyl)(amino)carbene (CAAC)-supported dihydroboron triflate yields the mixed doubly base-stabilised dihydroboryl cations [(CAAC)BH2L]+. Of these, [(CAAC)2BH2]OTf (OTf = triflate) underwent facile two-electron reduction with KC8 owing to a 1,2-hydride migration from boron to the carbene carbon to yield a stable hydroboryl anion. One-electron oxidation of the latter yielded the first neutral hydroboryl radical, which is bench-stable in the solid state.


 Please wait while we load your content...
Please wait while we load your content...